Latest Lab News
We found a novel flu RNA that activates RIG-I! Check out Liza's preprint!
Caitlin's Cas13 manuscript has been accepted in RNA and Kimmie's published in Virus Evol!
Kaleigh Remick was awarded a 2024 NSF fellowship!
Emmanuelle successfully defended her PhD thesis, January 18 2024!
Check out these exciting preprints from Ben, and our collaborations with Mathilde Richard and Alistair Russell.
Charlotte successfully defended her PhD thesis, December 11 2023!
Kimmie's review was published in MMBR!
Karishma received an LSRF fellowship!
Elizaveta successfully defended her PhD thesis, March 13 2023!
Caitlin Lamb was awarded a 2023 NSF fellowship!
Karishma's review was published in mBio!
Hollie's and Emmanuelle's paper was published in Science Advances!
AJ was awarded an NIH DP2 with impact score 10!
Job Openings
- We currently have no open/funded postdoc positions.
- Interested graduate students: students should apply via the MolBio graduate program. For general Departmental information, please find more information here.
- Interested undergraduate students: Princeton juniors should email for JP work starting in the spring semester. Other students can apply via the MOL280/1 course. We currently have no open positions for external students.
What We Do
We study how RNA viruses amplify, mutate, and cause disease
In 1918, the Spanish Flu killed 50-100 million people. Unfortunately, equally devastating viruses can emerge at any moment. In the 21st century, we have already seen outbreaks of the Ebola virus, avian influenza (H5N1 and H7N9), coronaviruses (SARS-CoV, SARS-CoV-2 and MERS-CoV) and Zika. In addition, viruses are costly: influenza alone costs the USA $90 billion per year. Clearly, we need to gain a better understanding of how these viruses amplify themselves in humans and how they cause disease.
The amplification of an RNA virus relies on the activity of an enzyme called the RNA polymerase (te Velthuis et al, Nature Rev Microbiol 2021). We use biochemical, virological, structural and single-molecule techniques to study how this viral enzyme works (an engineering problem) and how its activity contributes to the ability of RNA viruses to cause disease (a cell biology/virology/immunology problem). In addition, we study how inhibitors of viral replication work.
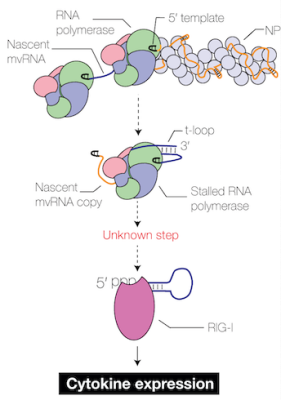
One of our key recent findings shows that the RNA polymerase from highly pathogenic viruses makes a lot of short aberrant RNA molecules, which we call mini viral RNAs (te Velthuis et al, Nature Microbiol 2018). A subset of the these mini viral RNAs contains an RNA structure that contributes to activation of the innate immune response (French et al, Science Advances 2022).
We are currently working on methods to screen for innate immune-inducing mini viral RNAs in patients, figuring our how they are made and if we can inhibit this, and we are investigating how they interact exactly with our immune response.
Lab Space
Lewis Thomas Laboratory 101


Virus room

Funding
Active funding
- NIH R01 AI170520
- NIH R01 AI177487
- NIH DP2 AI175474
- Princeton Catalysis Initiative
- Princeton Dean for Research
- Center for Health and Wellbeing
- NSF (to Caitlin Lamb and Kaleigh Remick)
- LSRF (to Karishma Bisht)
Previous funding
- Wellcome Trust Henry Dale 206579/Z/17/Z
- NIH R21 AI147172
- Royal Society grant
- Newton Trust grant
- Biochemical Society grant
- Microbiology Society grant
- Dutch Organization for Health Research (ZonMw)
- Dutch Organization for Scientific Research (NWO)
Green Lab
Research laboratories produce a lot of waste and use a lot of resources to run. My Green Lab has helped us become more aware of what we consume and produce, and we have tried to make an effort to reduce both! For our efforts, we received a Platinum Certificate based on our implementation of 78% of the Green Lab actions! We are aiming for >80% now!

Key Papers
- French H. & Pitre E. et al. Transient RNA structures cause aberrant influenza virus replication and innate immune activation. Science Advances. 8: eabp8655 (2022).
- te Velthuis A.J.W. et al. Structural insights into polymerases of negative strand RNA viruses. Nature Rev Microbiol. 19:220 (2021)
- Robb N.C. et al.: Real-time analysis of influenza virus replication initiation reveals dynamic differences between the vRNA and cRNA promoters. Nucleic Acids Res. 47:6466-6477 (2019).
- Goldhill D.H. et al.: The mechanism of resistance to favipiravir in influenza. PNAS USA pii:201811345 (2018).
- Te Velthuis A.J.W.: Mini viral RNAs act as innate immune agonists during influenza virus infection. Nature Microbiol. 3:1234-1242 (2018).
- Te Velthuis A.J.W. et al.: Influenza virus RNA polymerase: insights into the mechanisms of viral RNA synthesis. Nature Rev Microbiol. 14: 479-493 (2016)
- Te Velthuis A.J.W. et al.: The role of the priming loop in influenza A virus RNA synthesis. Nature Microbiol. 1:16029 (2016)












